The Two-Step Healing Process: Beyond Reducing IL-6
At Leading Edge Clinic, we recognize that bringing down IL-6 and reducing chronic inflammation is only the first step in recovery from Long COVID and Post-Vaccine Syndrome. True healing requires a second, equally crucial step: helping cells that have become trapped in a protective state to complete their healing cycle and return to normal function.
This two-step approach is grounded in cutting-edge research on cellular biology, specifically the Cell Danger Response and cellular senescence—two interconnected mechanisms that, when dysregulated, trap the body in a state of chronic illness.
Step 1: Reducing Inflammation and IL-6
As we’ve discussed, the first step involves addressing the chronic inflammatory state characterized by elevated IL-6, IL-8, and other pro-inflammatory markers. This includes pharmaceutical interventions, natural anti-inflammatory compounds, and lifestyle modifications that normalize the immune response and reduce systemic inflammation.
Step 2: Releasing Cells from the Danger Response
But reducing inflammation alone isn’t enough. Even after inflammatory markers improve, many patients continue to experience symptoms because their cells remain stuck in a protective, metabolically-altered state. This is where understanding the Cell Danger Response becomes critical.
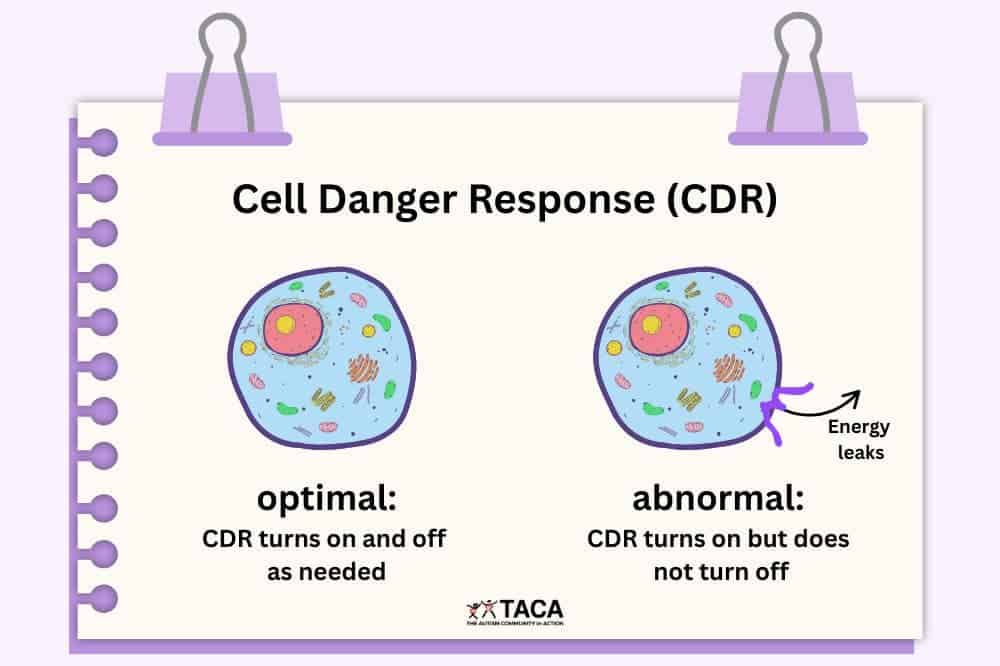
The Cell Danger Response: When Healing Gets Stuck
The Cell Danger Response (CDR) is a universal cellular response discovered and characterized by Dr. Robert Naviaux at UC San Diego. It’s a protective mechanism that all cells use when they sense threat or injury—whether from infection, toxins, physical trauma, or psychological stress.
How the Cell Danger Response Works
When cells detect danger, they undergo dramatic metabolic changes orchestrated primarily by mitochondria (the cell’s energy-producing structures):
- Metabolic shift: Cells switch from efficient energy production to a defensive, pro-inflammatory state
- ATP release: Damaged cells release ATP (the cell’s energy currency) into the extracellular space, signaling danger to neighboring cells
- Purinergic signaling: Extracellular ATP triggers a cascade of protective responses throughout the tissue
- Cellular isolation: Cells reduce their communication and cooperation with neighbors to prevent spreading the threat
In normal healing, this protective state is temporary. Once the threat is eliminated, cells progress through three distinct phases of the healing cycle: inflammation, proliferation, and differentiation, ultimately returning to their normal, healthy state.
When the CDR Persists: The Root of Chronic Illness
In Long COVID, Post-Vaccine Syndrome, and many other chronic illnesses, something goes wrong with this healing cycle. As Dr. Naviaux explains in his landmark 2018 paper, “Metabolic features and regulation of the healing cycle”:
“Chronic disease results when cells are caught in a repeating loop of incomplete recovery and re-injury, unable to fully heal. This biology is at the root of virtually every chronic illness known.”
The CDR was meant to be temporary—lasting days to weeks as part of normal healing. But when cells remain stuck in the danger response for months or years, they:
- Continue producing inflammatory signals even after the original threat is gone
- Maintain a metabolically-altered state that generates fatigue and dysfunction
- Fail to communicate properly with neighboring cells, disrupting tissue and organ function
- Create a vicious cycle where the prolonged danger state itself becomes a source of ongoing cellular stress
This explains why many Long COVID and Post-Vaccine Syndrome patients continue to struggle even after tests show inflammation has decreased: their cells haven’t received the signal that it’s safe to return to normal function.
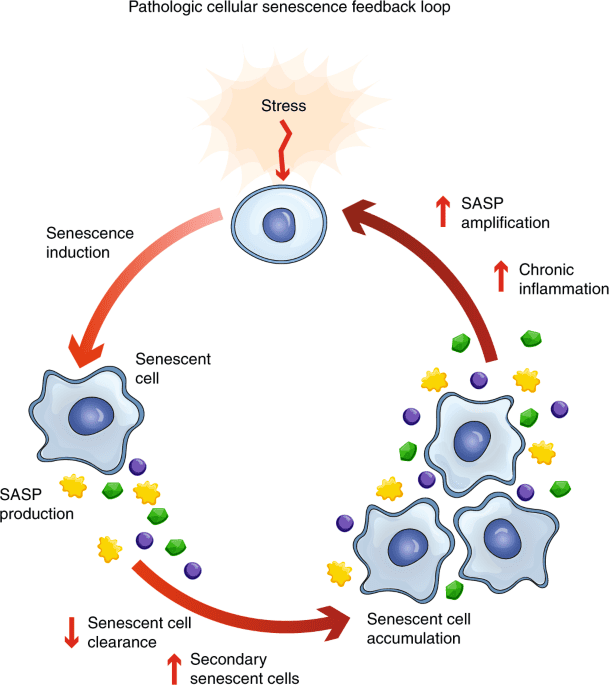
Cellular Senescence: The Inflammation-Driven Trap
Compounding the problem of the Cell Danger Response is another cellular state called senescence—and chronic inflammation like that seen in Long COVID and Post-Vaccine Syndrome is a direct driver of this process.
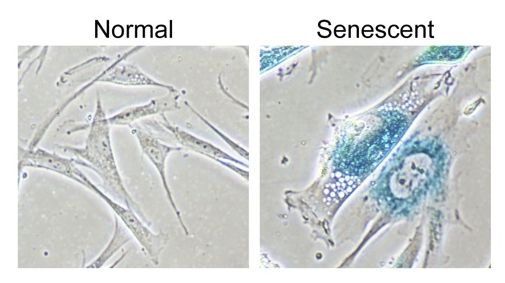
What Are Senescent Cells?
Cellular senescence is a state of permanent growth arrest that cells enter in response to stress or damage. First described in the 1960s by Leonard Hayflick and Paul Moorhead, senescent cells are characterized by:
- Irreversible cell cycle arrest: The cells stop dividing but don’t die
- Resistance to apoptosis: These cells evade programmed cell death, persisting abnormally
- SASP activation: They develop a Senescence-Associated Secretory Phenotype, releasing massive amounts of pro-inflammatory factors
- Altered metabolism: Increased glycolysis and reactive oxygen species production
- Molecular markers: Elevated p16 and p21 expression, decreased telomere length, increased SA-β-galactosidase activity
Originally, senescence was thought to be primarily a protective mechanism against cancer—damaged cells permanently stop dividing rather than becoming malignant. However, research now shows that when senescent cells accumulate in tissues, they become profoundly harmful.
The Senescence-Associated Secretory Phenotype (SASP)
The most damaging feature of senescent cells is SASP—a complex cocktail of inflammatory and bioactive molecules they continuously secrete, including:
- Pro-inflammatory cytokines: IL-6, IL-8, IL-1β (sound familiar?), TNF-α
- Chemokines: CXCL1, CXCL2 that recruit immune cells and amplify inflammation
- Proteases: Matrix metalloproteinases that degrade tissue structure
- Growth factors: That can paradoxically promote tumor growth in surrounding tissues
Here’s the critical connection: the same IL-6 elevation we see in Long COVID and Post-Vaccine Syndrome is both a marker of senescent cells and a driver that creates more senescent cells. It’s a vicious, self-perpetuating cycle.
How Chronic Inflammation Drives Cellular Senescence
Multiple pathways connect chronic inflammation to cellular senescence:
- DNA damage response: Persistent inflammation causes oxidative stress and DNA damage, triggering senescence pathways via p53 and p16INK4a/Rb activation
- NF-κB pathway activation: Pro-inflammatory cytokines like IL-6 activate NF-κB transcription factors, which upregulate SASP components and reinforce senescence
- Mitochondrial dysfunction: Chronic inflammation impairs mitochondrial function, generating excess reactive oxygen species (ROS) that damage cellular components and trigger senescence
- Telomere shortening: Oxidative stress from inflammation accelerates telomere erosion, a known senescence trigger
- Paracrine senescence: Senescent cells can induce senescence in neighboring healthy cells through SASP factors—creating a spreading wave of cellular dysfunction
A 2023 review in Signal Transduction and Targeted Therapy describes this relationship:
“Cellular senescence, initially identified as a protective mechanism to prevent the proliferation of damaged or stressed cells, has emerged as a key contributor to the chronic inflammation observed in aging, commonly referred to as ‘inflammaging.’ Senescent cells secrete a complex mixture of proinflammatory and bioactive molecules collectively known as the SASP.”
Research published in Nature Communications (2018) demonstrated that when the immune system fails to clear senescent cells efficiently, they accumulate in tissues, driving age-dependent chronic inflammation. This creates a bidirectional relationship:
- Chronic inflammation → Creates senescent cells
- Senescent cells → Produce more inflammation via SASP
- More inflammation → Creates more senescent cells
This self-reinforcing loop helps explain why post-viral syndromes can be so persistent and difficult to treat with conventional approaches.
The Link to Cancer and Accelerated Aging
The accumulation of senescent cells is not just about current symptoms—it’s a driver of both cancer and accelerated biological aging:
- Cancer promotion: While senescence prevents damaged cells from becoming cancerous, SASP factors create a pro-tumorigenic microenvironment that can promote cancer in neighboring cells. The chronic inflammation, growth factors, and tissue remodeling driven by SASP facilitate tumor development and progression.
- Accelerated aging: Senescent cells are found at sites of age-related pathologies including atherosclerosis, osteoarthritis, neurodegeneration, and organ fibrosis. Their accumulation drives the functional decline we associate with aging.
- Tissue dysfunction: Even a relatively small number of senescent cells (1-5% of tissue) can have profound effects on tissue function through their inflammatory secretions and disruption of normal cellular communication.
A 2021 review in Frontiers in Cell and Developmental Biology noted:
“Multiple studies have shown that most of the age-related pathologies stem from low level chronic inflammation referred to as inflammaging or sterile inflammation which can also result in premature aging. Therefore, SASP mediated autocrine and paracrine signaling may explain how a relatively small number of senescent cells can bring about durable, local and systemic effects in vivo, which promote chronic diseases and age-associated functional decline.”
The Root Cause: Spike Protein Persistence Triggers Cell Danger Response and Senescence
Now that we understand how chronic inflammation drives cellular senescence and how the Cell Danger Response traps cells in a dysfunctional state, a critical question emerges: What initially triggers this cascade in Long COVID and Post-Vaccine Syndrome?
The answer lies in spike protein persistence—the lingering presence of SARS-CoV-2 spike protein in tissues long after acute infection or vaccination. Recent research has established that spike protein itself directly damages mitochondria, triggers the Cell Danger Response, and induces cellular senescence, creating the perfect storm for chronic post-viral illness.
Spike Protein Directly Attacks Mitochondria: The CDR Trigger
Groundbreaking research from the Salk Institute, published in Circulation Research (2021), demonstrated something remarkable: the spike protein alone—without any viral replication—is sufficient to cause significant cellular damage.
The research team created a ‘pseudovirus’ containing spike proteins but no viral genetic material. When exposed to this pseudovirus:
- Animals developed lung damage and vascular injury
- Endothelial cells (lining blood vessels) showed inflammation and dysfunction
- Mitochondria became fragmented and dysfunctional
- ACE2 signaling to mitochondria was disrupted
As lead researcher Dr. Uri Manor explained: “If you remove the replicating capabilities of the virus, it still has a major damaging effect on the vascular cells, simply by virtue of its ability to bind to this ACE2 receptor.”
This finding is crucial: spike protein persistence, not ongoing viral infection, can drive chronic pathology.
Mitochondrial Dysfunction Activates the Cell Danger Response
Multiple studies have now documented the specific ways spike protein damages mitochondria—and these mechanisms directly map onto Dr. Naviaux’s Cell Danger Response pathway:
1. Mitochondrial Fragmentation and Disrupted Energy Production
Research published in Cells (2023) examining human cardiomyocytes found that the S1 spike protein subunit:
- Disrupts mitochondrial membrane potential (Δψm)
- Causes mitochondrial calcium overload
- Reduces ATP production (after initial 24h increase)
- Increases mitochondrial fragmentation and fission
- Downregulates TOM20, inhibiting mitochondrial biogenesis
These are precisely the mitochondrial changes that define the Cell Danger Response: damaged mitochondria switching from efficient energy production to a defensive, pro-inflammatory state.
2. Reactive Oxygen Species (ROS) Production and Oxidative Stress
The Journal of Neuroimmune Pharmacology (2021) studied spike protein effects on human microglial cells (brain immune cells) and found:
- Increased mitochondrial oxygen consumption rate (OCR)
- Massive ROS production and oxidative stress
- Mitochondrial morphology changes indicating stress
- Activation of apoptotic pathways
Critically, the researchers noted: “mitochondrial DNA itself acts as a danger-associated molecular pattern (DAMP) and mitochondrial dysfunction drives a systemic immune response.”
This is a direct description of the Cell Danger Response—damaged mitochondria releasing danger signals (DAMPs including mtDNA) that trigger and sustain inflammatory responses.
3. Extracellular ATP Release: The Danger Signal
When mitochondria are damaged by spike protein, cells release ATP into the extracellular space. As Dr. Naviaux’s research established, extracellular ATP (eATP) is the primary danger signal that triggers and maintains the Cell Danger Response.
The spike protein’s assault on mitochondria creates exactly the conditions for persistent eATP elevation—damaged membranes leaking ATP, dysfunctional energy production, ongoing cellular stress. This sustained purinergic signaling keeps cells locked in danger mode, unable to progress through the healing cycle.
Spike Protein Directly Induces Cellular Senescence
Beyond triggering mitochondrial dysfunction and the Cell Danger Response, spike protein has been shown to directly induce cellular senescence—the second phase of the dysfunction we discussed earlier.
Study: Spike Protein Causes Paracrine Senescence
A study published in 2021 titled “SARS-CoV-2 Spike Protein Induces Paracrine Senescence and Leukocyte Adhesion in Endothelial Cells” demonstrated that spike protein expression in epithelial cells creates a senescent phenotype that spreads to neighboring cells:
Direct senescence markers: Cells exposed to spike protein showed increased p16, p21, and SA-β-galactosidase expression—the classic markers of cellular senescence
SASP activation: Spike protein triggered release of SASP factors including IL-6, IL-8, and inflammatory molecules
Paracrine senescence: Culture medium from spike-expressing cells induced senescence in healthy endothelial cells—demonstrating the spreading effect
Therapeutic intervention: Treating cells with an IL-6 inhibitor prevented spike-induced senescence, confirming IL-6’s role
This study reveals the mechanism by which spike protein persistence creates expanding zones of cellular dysfunction—senescent cells producing SASP factors that induce senescence in neighboring healthy cells, creating a self-propagating wave of cellular aging and inflammation.
Confirmation in Brain Cells: TLR7-Mediated Senescence
A 2025 study in Journal of Neuroinflammation examined spike protein effects on human astrocytes (brain support cells) and uncovered the cellular mechanism:
- S1 spike protein enters cells and localizes to endolysosomes
- Triggers TLR7 (Toll-like receptor 7), an endolysosome danger sensor
- Causes endolysosome dysfunction and cellular stress
- Results in increased IL-6 release, p16, p21, and SA-β-gal expression
- Creates lasting cellular senescence even from transient spike protein exposure
The researchers noted this mechanism helps to partially explain neurological symptoms in Long COVID (and Post-Vaccine Syndrome), as senescent astrocytes can no longer properly support neuronal function.
Both Variants Cause Senescence
Research published in Frontiers in Cellular and Infection Microbiology (2024) tested both ancestral and Omicron spike proteins and found:
- Both variants induce cellular senescence markers
- Both increase SA-β-gal positive cells, p16, p21, and SASP factors
- Omicron spike may actually induce higher p16/p21 expression than ancestral (despite the acute phase of infection being less severe)
- Effects are dose-dependent—more spike protein = more senescence
This finding is significant for Long COVID and Post-Vaccine patients who may have been reinfected with different variants, and helps explain why symptoms can persist or worsen with reinfection.
Emerging Evidence on Recent Variants
Leading Edge Clinic maintains close collaborative relationships with research institutions studying post-viral synescence, including a German laboratory specializing in the development of spike protein detoxification products. Their preliminary laboratory findings suggest that the most recent SARS-CoV-2 variants may demonstrate an even greater capacity to induce cellular senescence compared to earlier strains. While this laboratory data awaits peer review and publication, it aligns with the published trajectory shown in the 2024 Frontiers in Cellular and Infection Microbiology study, which found that Omicron spike protein induced higher p16 and p21 expression than ancestral variants. This suggests that evolutionary changes in the spike protein may be progressively increasing its senescence-inducing properties. You will note that the acute viral phases have become less severe, but these findings would indicate the risk of post-viral syndromes arising is increasing, which is confirmed by our own observations within our clinic.
These preliminary findings would help explain why some patients report more severe or persistent Long COVID symptoms following infection with recent variants. Leading Edge Clinic maintains close collaborative relationships with research institutions investigating these mechanisms, ensuring our treatment protocols remain current with emerging science. Regardless of which variant caused infection, addressing spike protein persistence and cellular senescence remains critical for recovery—and may be even more important for patients infected with newer strains.
Why Both Steps Are Essential for Recovery
Now you can see why a two-step approach is essential:
If you only reduce IL-6 and inflammation (Step 1):
- Cells may remain stuck in the Cell Danger Response
- Senescent cells persist, continuing to secrete SASP factors
- Symptoms improve but don’t fully resolve
- Relapses occur easily when you encounter new stressors
If you only try to reset the Cell Danger Response (Step 2) without addressing inflammation:
- The inflammatory environment keeps re-triggering the danger response
- New senescent cells continue forming
- The body cannot exit the danger state sustainably
Both steps must work together: reduce the inflammatory triggers while simultaneously helping cells complete their healing cycle and clearing senescent cells that perpetuate the problem.
Leading Edge Clinic’s Two-Step Approach
At Leading Edge Clinic, our comprehensive treatment protocols address both phases of the healing process:
Phase 1: Reducing Inflammation and IL-6
As described in last week’s blog post, this includes:
- Anti-inflammatory pharmaceuticals and nutraceuticals
- IL-6 pathway targeted interventions
- Lifestyle and dietary modifications
- Addressing root causes like spike protein persistence and microclotting
Phase 2: Releasing the Cell Danger Response and Addressing Senescence
Purinergic signaling modulation:
- Low-dose naltrexone (LDN): May help modulate danger signaling and promote healing cycle progression
- Improve Redox Homeostasis: A balanced redox state ensures proper cellular communication, inflammation control, and energy regulation.
Mitochondrial restoration:
- Improving Cellular Coherence: optimized communication, structural integrity, and energy harmony within and between cells reduces oxidative stress and fosters efficient energy production
- Improve Redox Homeostasis: reducing oxidative stress, protecting against damage to mtDNA and lipids, and regulating ATP production
- Microcurrent Therapy: mimics the body’s natural bioelectric signals, boosting ATP production by up to 500%
- Nutraceutical Therapies: supporting with biovailable micronutrients depleted by chronic illness to support energy production, biogenesis, antioxidant support, cell rapir, and mitochondrial membrane repair
Senolytic interventions (clearing senescent cells):
- Quercetin + Fisetin combination: Natural senolytic compounds that selectively induce apoptosis in senescent cells. Fisetin in particular has shown promise in clearing senescent cells.
- Intermittent fasting and autophagy promotion: Autophagy is the body’s cellular recycling program that helps clear dysfunctional cells and components; however, while autophagy can help clean up the affected cells surrounding senescent cells, it may not always be able to get to the senescent cells at the root of the problem
- Spermidine: A polyamine that induces autophagy and may help clear senescent cells
- Resveratrol: Activates sirtuins and AMPK pathways involved in cellular cleanup
Senomorphic interventions (reducing SASP without killing cells):
- Metformin: Beyond its anti-diabetic effects, metformin can reduce SASP factor secretion from senescent cells
- Rapamycin (low-dose, intermittent): mTOR inhibition can reduce SASP and promote autophagy (used cautiously due to immunosuppressive effects)
Supporting the healing cycle progression:
- Adequate sleep: Sleep is when the body does most of its cellular repair and healing cycle progression
- Stress reduction: Chronic stress keeps cells in danger mode; parasympathetic activation helps complete healing
- Gentle movement: Within energy limits, appropriate movement supports metabolic flexibility and healing
- Nutrient sufficiency: Cells need adequate building blocks (amino acids, essential fatty acids, vitamins, minerals) to complete the healing cycle
The Timeline: Understanding That Deep Healing Takes Time
It’s important to set realistic expectations. Phase 1 (reducing inflammation) may show measurable improvements in weeks to months. However, Phase 2 (releasing the Cell Danger Response and clearing senescent cells) is a deeper, non-linear process that can take 6-18 months or longer.
As Dr. Naviaux notes in his 2023 paper on “Mitochondrial and metabolic features of salugenesis and the healing cycle”:
“Abnormal persistence of any phase of the CDR inhibits the healing cycle, creates dysfunctional cellular mosaics, causes the symptoms of chronic disease, and accelerates the process of aging. When chronic pain, disability, or disease is established, salugenesis-based therapies will start where pathogenesis-based therapies end.”
This is why our approach at Leading Edge Clinic focuses not just on treating disease (pathogenesis), but on promoting healing (salugenesis)—a fundamentally different paradigm that recognizes healing as an active, resource-consuming, genetically-programmed process that requires specific support.
Conclusion: A Complete Framework for Understanding and Healing
The discovery that IL-6 elevation drives both immediate symptoms and long-term cellular dysfunction through senescence and the Cell Danger Response provides a complete framework for understanding why Long COVID and Post-Vaccine Syndrome are so persistent—and how to effectively treat them.
The two-step healing process is not just a theory—it’s grounded in mitochondrial biology (and physics) and senescence research that explains:
- Why reducing inflammation alone isn’t enough
- Why cells can remain dysfunctional even after inflammatory markers improve
- Why true healing requires both addressing the inflammatory triggers and helping cells complete their healing cycle
- Why the timeline for recovery is measured in months to years, not weeks
- Why addressing these conditions now protects against accelerated aging and cancer risk in the future
At Leading Edge Clinic, we’re committed to translating this complex biology into practical, effective treatment protocols that address both phases of healing. We don’t just chase symptoms—we work to restore normal cellular function, complete the healing cycle, and protect your long-term health. If you are interested in being treated for Long Haul Covid or Post-Vaccine Syndrome by our expert clinicians, you can register here to become a patient
Important Information About This Article
We’ve created this comprehensive guide to help you understand the latest research on Long COVID and Post-Vaccine Syndrome. However, this article is educational in nature and not a substitute for personalized medical care.
Every patient’s situation is unique. While the research and treatment approaches discussed here are based scientific studies and clinical experience, your individual health needs require evaluation by qualified healthcare professionals who can:
- Review your complete medical history and current health status
- Order appropriate diagnostic testing
- Consider potential drug interactions with your current medications
- Monitor your response to treatment and adjust protocols accordingly
- Coordinate care with your other healthcare providers
Please do not start any new treatments, supplements, or make changes to your current medical regimen without first consulting with a healthcare provider. If you’re experiencing severe symptoms or a medical emergency, seek immediate medical attention.
At Leading Edge Clinic, we’re committed to translating cutting-edge research into practical treatments, but we can only do that effectively through proper clinical evaluation and ongoing monitoring. We encourage you to share this information with your healthcare team as part of informed discussions about your care, or join our clinic as a patient to receive the most up-to-date and expert care in Long Haul Covid, Post-Vaccine Syndrome, and other Complex Medical Conditions.
Additional Key References for the Two-Step Healing Process:
- Naviaux RK. Metabolic features of the cell danger response. Mitochondrion. 2014 May;16:7-17.
- Naviaux RK. Metabolic features and regulation of the healing cycle-A new model for chronic disease pathogenesis and treatment. Mitochondrion. 2018.
- Naviaux RK. Perspective: Cell danger response Biology-The new science that connects environmental health with mitochondria and the rising tide of chronic illness. Mitochondrion. 2020 Mar;51:40-45.
- Naviaux RK. Mitochondrial and metabolic features of salugenesis and the healing cycle. Mitochondrion. 2023 May;70:131-163.
- Naviaux RK. Incomplete Healing as a Cause of Aging: The Role of Mitochondria and the Cell Danger Response. Biology. 2019 May;8(2):27.
- Coppé JP, et al. Inflammatory Networks during Cellular Senescence: Causes and Consequences. PMC. 2010.
- Signal Transduction and Targeted Therapy. Inflammation and aging: signaling pathways and intervention therapies. June 2023.
- Nature Communications. Impaired immune surveillance accelerates accumulation of senescent cells and aging. December 2018.
- Frontiers in Cell and Developmental Biology. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. February 2021.
- Journal of Cell Biology, Rockefeller University. Senescence and aging: Causes, consequences, and therapeutic avenues. January 2018.
- Experimental & Molecular Medicine. Targeting immunosenescence and inflammaging: advancing longevity research. September 2025.
- Bone Research. Cross-talk of inflammation and cellular senescence: a new insight into the occurrence and progression of osteoarthritis. December 2024.