Understanding Drug Repurposing in Modern Oncology
Cancer treatment continues to evolve beyond traditional chemotherapy and radiation. One promising frontier is drug repurposing—using established medications with known safety profiles for new therapeutic applications. Among these repurposed agents, ivermectin has emerged as a compelling option for adjunctive cancer therapy, backed by robust preclinical research and growing clinical evidence.
Originally developed as an antiparasitic medication, ivermectin earned its discoverers the 2015 Nobel Prize in Physiology or Medicine (Crump & Ōmura, 2011). Today, researchers are uncovering its potential to complement conventional cancer treatments through multiple anticancer mechanisms that target the very pathways cancer cells rely on for survival (Tang et al., 2021; Juarez et al., 2018).
What Makes Ivermectin a Promising Anticancer Agent?
The Science Behind Ivermectin's Anticancer Properties
Ivermectin’s effectiveness against cancer stems from its ability to disrupt multiple cellular processes that tumors depend on. Unlike conventional chemotherapy agents that typically target one pathway, ivermectin acts as a “multi-targeted” drug, simultaneously affecting several cancer hallmarks.
Key Mechanisms of Action
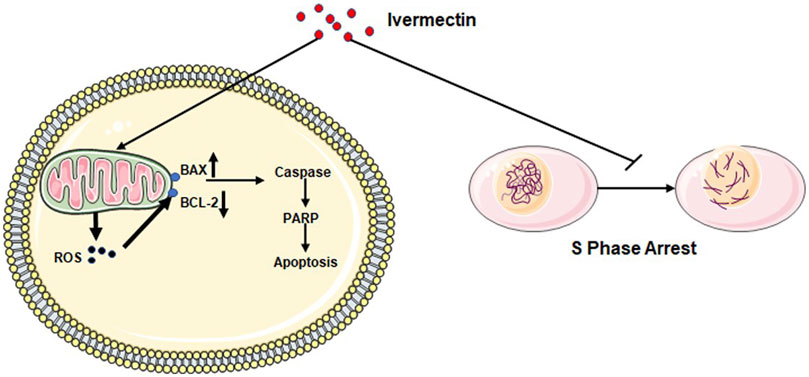
- Autophagy Induction Through PAK1/Akt/mTOR Pathway Blockade: One of ivermectin’s most well-documented anticancer mechanisms involves promoting cytostatic autophagy. Research demonstrates that ivermectin promotes the degradation of PAK1 (P21-activated kinase 1) through ubiquitination, which subsequently blocks the Akt/mTOR signaling pathway—a critical regulator of cell growth and survival in cancer (Dou et al., 2016; Wang et al., 2016). When this pathway is inhibited, cancer cells undergo excessive autophagy, essentially self-digesting beyond their capacity to survive. Importantly, this process preferentially affects cancer cells while largely sparing healthy tissue, as studies show ivermectin cannot significantly stimulate autophagy in normal breast cells at therapeutic concentrations (Dou et al., 2016; Wang et al., 2016).
- Mitochondrial Dysfunction and Energy Depletion: Cancer cells have notoriously high energy demands to fuel their rapid proliferation. Ivermectin inhibits mitochondrial complex I in the electron transport chain, dramatically reducing ATP production—the energy currency cells need to function (Tang et al., 2021). This metabolic collapse triggers oxidative stress, damages cellular components, and ultimately accelerates cancer cell death through apoptosis.
- Cancer Stem Cell Targeting: Perhaps one of ivermectin’s most significant advantages is its ability to target cancer stem cells (CSCs)—the subpopulation of cells responsible for tumor recurrence, metastasis, and treatment resistance. Research shows ivermectin preferentially inhibits CSC-enriched populations compared to bulk tumor cells, downregulating key stemness genes including NANOG, SOX2, and OCT4 (Dominguez-Gomez et al., 2018; Napier et al., 2020). In breast cancer studies, ivermectin demonstrated superior activity against CD44+/CD24- stem-like cell populations—the very cells that drive tumor regrowth after conventional therapy (Dominguez-Gomez et al., 2018). This CSC-targeting capacity addresses one of oncology’s greatest challenges: preventing relapse after initial treatment.
- WNT/β-Catenin Pathway Inhibition: The WNT signaling pathway plays a central role in cancer development, particularly in colorectal, breast, and lung cancers. Ivermectin blocks WNT-TCF pathway responses by affecting β-catenin function and phosphorylation status (Melotti et al., 2014). Studies demonstrate that ivermectin suppresses positive WNT regulators (AXIN2, LGR5, ASCL2) while promoting pathway repressors like FILIP1L (Melotti et al., 2014). This inhibition reduces cancer cell proliferation, suppresses epithelial-to-mesenchymal transition (EMT)—a process critical for metastasis—and decreases the expression of metastasis-related proteins such as vimentin and snail (Rujimongkon et al., 2025).
- Anti-Metastatic Effects: Metastasis accounts for approximately 90% of cancer deaths, making anti-metastatic therapies critically important. Ivermectin inhibits tumor metastasis through multiple mechanisms (Jiang et al., 2022):
- Suppressing the Wnt/β-catenin/integrin β1/FAK signaling cascade
- Reducing matrix metalloproteinase-9 (MMP-9) expression
- Inhibiting cancer cell migration and invasion
- Preventing epithelial-to-mesenchymal transition
Animal studies confirm these findings, with ivermectin significantly reducing tumor metastasis in xenograft models without causing significant toxicity (Jiang et al., 2022):.
-
Synergy with Standard Cancer Treatments: Ivermectin enhances the effectiveness of conventional cancer therapies. Research demonstrates synergistic effects when combined with:
- Targeted therapies (sorafenib in hepatocellular carcinoma, osimertinib in EGFR-positive lung cancer) (Lu et al., 2022)
- Chemotherapy agents (docetaxel, cyclophosphamide, tamoxifen, carboplatin) (Juarez et al., 2018)
- Immune checkpoint inhibitors (pembrolizumab, balstilimab) (Yuan et al., 2022)
These combinations often achieve superior tumor control compared to standard treatments alone, potentially at lower doses with reduced toxicity (Lu et al., 2022).
The Evidence Base: From Laboratory to Clinical Practice
Extensive in vitro and animal studies demonstrate ivermectin’s anticancer activity across multiple cancer types:
- Breast Cancer: Inhibits growth through PAK1/Akt/mTOR pathway blockade, particularly effective against triple-negative breast cancer (TNBC) and hormone-resistant subtypes (Dou et al., 2016; Rujimongkon et al., 2025)
- Lung Cancer: Induces nonprotective autophagy and apoptosis in both non-small cell lung cancer (NSCLC) and lung adenocarcinoma (Li et al., 2024)
- Colorectal Cancer: Blocks WNT-TCF signaling, suppresses proliferation and metastasis (Melotti et al., 2014; Jiang et al., 2022)
- Hepatocellular Carcinoma: Inhibits mTOR/STAT3 pathways, suppresses EMT, reduces stem cell marker expression (Lu et al., 2022)
- Pancreatic Cancer: Shows synergistic efficacy when combined with metabolic therapies (Hoffman et al., 2025)
- Ovarian Cancer: Demonstrates anti-proliferative effects through PAK1 inhibition (Hashimoto et al., 2009)
Pharmacokinetic Considerations
A critical question in translating laboratory findings to clinical use is whether therapeutic drug levels are achievable in humans. Studies in healthy volunteers show that ivermectin doses of 2 mg/kg produce plasma concentrations around 5-5.2 µM—levels that have demonstrated anticancer efficacy in preclinical studies (Guzzo et al., 2002; Lu et al., 2022). This suggests that clinically relevant anticancer activity is achievable within the established safety profile for parasitic infections.
Ongoing Clinical Trials & Observational Studies
Multiple registered clinical trials are currently investigating Ivermectin in cancer patients:
NCT05318469: A phase I/II trial at Cedars-Sinai Medical Center evaluating Ivermectin combined with immune checkpoint inhibitors (balstilimab or pembrolizumab) in metastatic triple-negative breast cancer (Yuan et al., 2022). This study is testing whether combining Ivermectin with immunotherapy can improve tumor shrinkage and progression-free survival in one of breast cancer’s most aggressive subtypes.
NCT02366884: A phase II trial testing “atavistic chemotherapy”—the concept that cancer cells behave like primitive organisms—using FDA-approved antimicrobial drugs including Ivermectin for advanced or metastatic cancers (Arguello Cancer Clinic, 2015).
WCG IRB #20240731: An observational conducted by Rebuild Medicine to evaluate the impact of repurposed drugs and metabolic therapies on the outcomes of patients with cancer. Leading Edge Clinic is the sole participating clinic in this study. What is different about this study is that it does not view Ivermectin in isolation. A little more on that in the following section…
Real-World Clinical Outcomes: Case Series from Leading Edge Clinic
Overview of Treatment Protocol
As mentioned, the observational study we are conducting does not look at Ivermectin in a vaccuum. Instead, Leading Edge Clinic is employing a combination of repurposed therapies, lifestyle changes, and supplements to address cancer. The goal is to cover as many signaling pathways as possible, giving patients the best chance, without putting them at risk. The following case series at Leading Edge Clinic demonstrates the real-world application of Ivermectin as part of comprehensive integrative oncology protocols in five Lung Cancer patients. All five patients received individualized combinations of:
- Repurposed medications: Ivermectin, Mebendazole, Metformin, Propranolol, Low-Dose Naltrexone, Doxycycline
- Metabolic interventions: Ketogenic diet
- Natural compounds: EGCG, melatonin, curcumin, omega-3 fatty acids, high-dose vitamin D, berberine
- Standard-of-care therapies where indicated
Case 1: Complete Remission in ALK-Positive NSCLC
Patient Profile: 60-year-old male with metastatic adenocarcinoma (ALK mutation)
Presentation: Progressive weakness, cough, weight loss; diagnosed May 2024 with metastatic non-small cell lung cancer
Treatment Approach: Alectinib (ALK inhibitor) combined with comprehensive integrative protocol including ivermectin, mebendazole, propranolol, itraconazole, metformin, ketogenic diet, and supportive supplements
Outcomes:
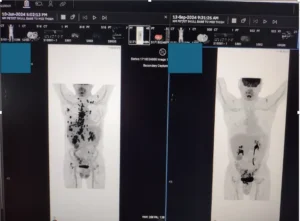
- September 2024 PET scan: Near-complete resolution of metastatic lung disease
- December 2024: Complete lung cancer remission; concurrent kidney lesion reduced from 5.0 cm to 4.1 cm
- Patient reduced alectinib dose due to fatigue while maintaining remission
Clinical Significance: Complete remission rates with alectinib alone average only 4.3% in published studies. The achievement of complete remission suggests substantial contribution from the integrative protocol, particularly given dose reduction of the targeted therapy.
Case 2: Disease Stability Without Standard Treatment
Patient Profile: 80-year-old male with EGFR-positive NSCLC
Presentation: Lung nodule detected during pneumonia treatment (March 2024), progressed to Stage IIIC adenocarcinoma by January 2025
Treatment Approach: Comprehensive repurposed drug protocol WITHOUT standard-of-care cancer therapy. Included ketogenic diet, ivermectin, mebendazole, high-dose vitamin D, propranolol, low-dose naltrexone, doxycycline, and supportive compounds.
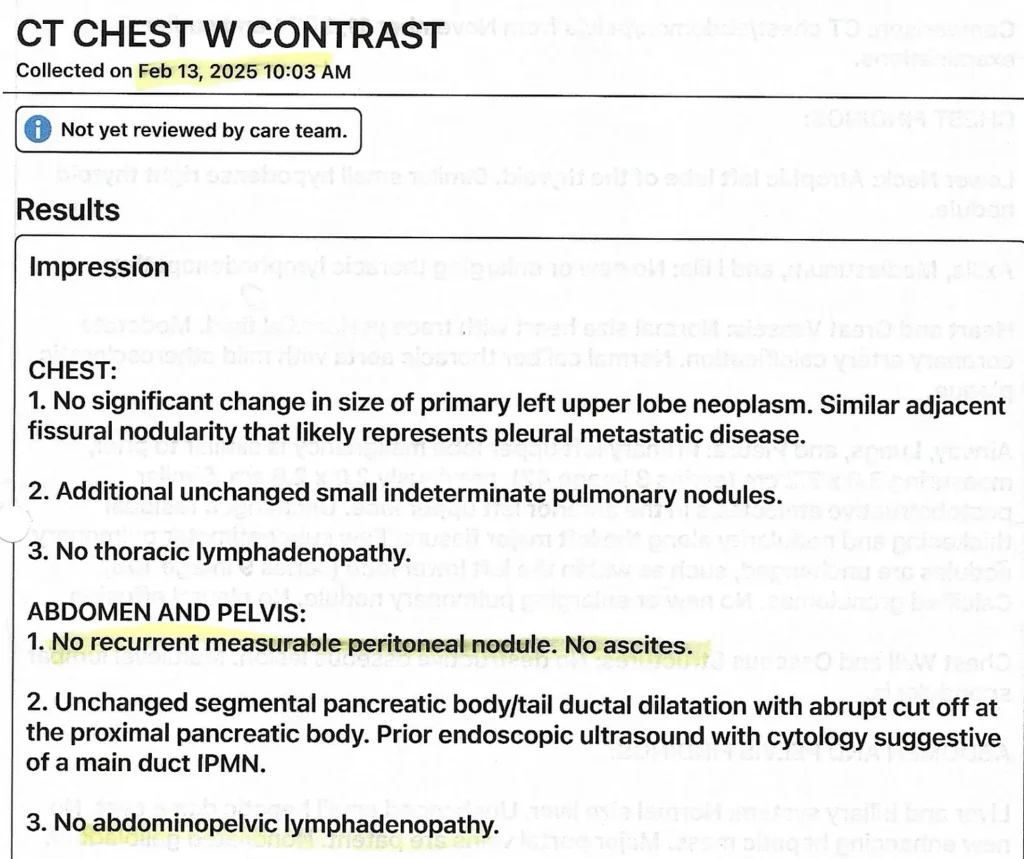
Outcomes:
- May 2025 PET scan: Stable lung mass (3.1 x 2.2 cm) with no new metastases over 6 months
- No lymph node enlargement or disease progression
- Survived 8 months since Stage IIIC diagnosis (16 months from first radiographic evidence)
Clinical Significance: AI analysis predicted median survival under 1 year for an 80-year-old refusing standard treatment. This patient achieved disease stability exclusively through metabolic and repurposed therapies, challenging conventional expectations for elderly patients with advanced disease.
Case 3: Stability in Aggressive Squamous Cell Lung Cancer
Patient Profile: 86-year-old male with Stage IV squamous cell lung cancer
Presentation: Originally Stage I disease treated with stereotactic radiation (2023); liver metastasis detected December 2024
Treatment Approach: Ivermectin, mebendazole, high-dose vitamin D, metformin, propranolol, doxycycline, plus radiation to liver metastasis and comprehensive supplement protocol
Outcomes:
- June 2025 PET scan: No disease progression over 7 months
- Patient remains active with only mild fatigue
- Maintains quality of life despite advanced age and aggressive cancer histology
Clinical Significance: Squamous cell lung cancer with liver metastases typically has poor prognosis. Seven months of stability in an 86-year-old patient highlights the efficacy of CSC-targeting repurposed drugs in aggressive disease.
Case 4: Sustained Control of Multifocal EGFR-Positive Disease
Patient Profile: 85-year-old male with Stage IV EGFR-positive adenocarcinoma
Presentation: Persistent cough led to diagnosis April 2024 with multifocal disease involving pleura
Treatment Approach: Osimertinib (Tagrisso) combined with comprehensive integrative protocol including ketogenic diet, ivermectin, mebendazole, high-dose vitamin D, propranolol, itraconazole, low-dose naltrexone, metformin, berberine, and full supplement regimen
Outcomes:
- Serial imaging August 2024-July 2025: Primary tumor and metastases reduced in size
- Much of lymph node involvement resolved
- No new metastases detected
- Patient maintains independence despite pleural involvement
Clinical Significance: EGFR inhibitors typically slow progression rather than resolve disease. The reduction in primary tumor size, resolution of lymph nodes, and absence of new metastases over 16 months demonstrates the profound contribution of metabolic and repurposed therapies.
Case 5: Quality of Life Preservation in Aggressive Disease
Patient Profile: 67-year-old male with biphasic lung tumor
Presentation: Initial left lung resection June 2022; recurrence in right lung June 2023, progressive by June 2024
Treatment History: Multiple chemotherapy regimens (Adriamycin, Taxotere, Gemzar, Navelbine) switched due to toxicities
Current Approach: Repurposed drug protocol (ivermectin, mebendazole, low-dose naltrexone, doxycycline) with targeted radiation to six nodules, plus carboplatin/etoposide chemotherapy
Outcomes:
- Radiated nodules showed shrinkage
- Untreated nodules showed growth (highlighting need for comprehensive coverage)
- Maintained stable weight and Karnofsky performance status 70-80
- Quality of life preserved despite aggressive disease
Clinical Significance: This case demonstrates both the benefits and limitations of partial protocol adherence (dietary non-compliance) while highlighting how integrative approaches can preserve quality of life during necessary chemotherapy.
Clinical Implications and Practical Considerations
Clinical Implications and Practical Considerations
Of five consecutive metastatic lung cancer patients:
- One achieved complete remission (with ALK inhibitor + integrative protocol)
- Three maintained stable disease (one with EGFR inhibitor, two exclusively with repurposed drugs)
- One experienced mixed response with preserved quality of life despite aggressive disease
Notably, three patients were in their 80s, a population typically excluded from clinical trials and often deemed “too frail” for aggressive treatment.
Key Success Factors
- Comprehensive Metabolic Approach: All protocols included ketogenic diet or metabolic optimization, recognizing cancer’s dependence on glucose metabolism
- Multi-Drug Synergy: Combining multiple repurposed agents targeting different pathways (proliferation, autophagy, angiogenesis, stemness, metastasis)
- Individualized Dosing: Careful titration based on tolerability, with dose adjustments to minimize side effects while maintaining efficacy
- Close Monitoring: Serial imaging, laboratory assessments, and nursing follow-up enabled timely adjustments
- Patient Autonomy: Shared decision-making respected patient preferences while optimizing medical management
Safety Profile and Adverse Effects
Ivermectin has an established safety record from decades of use in parasitic infections. In these cancer cases, most side effects were mild and manageable:
- Fatigue (most common, often improved with dose reduction)
- Hypercalcemia (when using high-dose vitamin D concurrently)
- Gastrointestinal effects (minimized with food intake and gradual titration)
- Somnolence (with certain combinations, resolved with discontinuation)
Serious adverse events were rare and typically related to chemotherapy rather than repurposed drugs.
Understanding the Limitations and Need for Further Research
While these clinical outcomes outlined in our case series are encouraging, several important limitations must be acknowledged:
- Small Sample Size: These five cases represent preliminary real-world evidence, not definitive proof of efficacy. We will publish findings for the hundreds of patients we have seen when the study is concluded. At that point, we hope the significant data we have generated will demonstrate proof of efficacy that can not be ignored.
- Multi-Drug Protocols: The simultaneous use of multiple agents makes it difficult to isolate individual drug contributions. However, we believe it is necessary to address as many cancer pathways as possible. Our goal is results for our patients, not proving any single drug’s efficacy.
- Selection Bias: Given this is an observational study, these patients actively sought integrative care and may differ from general populations
- Variable Adherence: Some patients struggled with dietary restrictions or experienced side effects requiring modifications
The Path Forward: Rigorous Clinical Investigation
Despite promising preliminary evidence, the oncology community needs:
- Large-scale randomized controlled trials comparing Ivermectin-based protocols to standard care
- Biomarker studies identifying which patients are most likely to benefit
- Optimal dosing studies determining the most effective dose-schedule combinations
- Mechanism validation confirming proposed mechanisms in human tumor samples
- Safety monitoring in larger populations, especially in combination with other cancer therapies
- Cost-effectiveness analyses evaluating the economic impact of repurposed drug strategies
Why Ivermectin Deserves Serious Scientific Attention
The Case for Investigation
1. Established Safety Profile: Decades of human use provide confidence in its tolerability
2. Multi-Targeted Activity: Affects multiple cancer hallmarks simultaneously, potentially reducing resistance development
3. CSC Targeting: Addresses one of oncology’s greatest challenges—cancer stem cells that drive relapse
4. Accessibility and Affordability: As a generic medication no longer under patent protection, Ivermectin could make effective cancer therapy more accessible globally
5. Synergy with Standard Treatments: Potential to enhance effectiveness of existing therapies, possibly allowing dose reduction and decreased toxicity
6. Preclinical Validation: Extensive laboratory evidence demonstrating anticancer mechanisms across multiple cancer types
7. Emerging Clinical Evidence: Growing number of case reports and case series showing clinical benefit
The Risk of Dismissal
The oncology community faces an important decision: continue dismissing ivermectin due to lack of large trials, or pursue rigorous investigation given accumulating evidence. History shows that some of our most important cancer discoveries came from observing unexpected effects of existing drugs.
Conclusion: A Paradigm Shift in Cancer Care?
The evidence presented here—from molecular mechanisms to clinical outcomes—suggests that Ivermectin deserves serious consideration as an adjunctive cancer therapy. The five cases from Leading Edge Clinic demonstrate that integrative protocols incorporating Ivermectin and other repurposed drugs can achieve outcomes ranging from complete remission to stable disease control, even in elderly patients with advanced disease who might otherwise have limited options.
These results challenge the traditional paradigm that new, expensive, targeted therapies are the only path to improved cancer outcomes. They suggest that thoughtful repurposing of existing medications, combined with metabolic interventions and careful monitoring, may offer a complementary strategy that expands the therapeutic armamentarium while potentially improving accessibility.
However, preliminary success must be balanced with scientific rigor. While these cases provide compelling real-world evidence and hypothesis generation for future studies, they do not replace the need for randomized clinical trials. The oncology community should view this evidence not as definitive proof, but as a call to action for properly designed clinical investigations.
For patients and healthcare providers considering Ivermectin as part of cancer treatment, several principles emerge from this analysis:
- Integration, not replacement: Repurposed drugs work best as part of comprehensive protocols that may include standard therapies
- Individualization is essential: Dosing and drug combinations should be tailored to each patient’s unique situation
- Close monitoring is mandatory: Regular imaging and laboratory work enable timely adjustments
- Realistic expectations: Not all patients will respond; success requires commitment to the full protocol
- Multidisciplinary care: Best outcomes involve collaboration between integrative and conventional oncology
The story of Ivermectin in cancer care is still being written. From its origins as a Nobel Prize-winning antiparasitic to its emerging role in oncology, this drug exemplifies the potential of drug repurposing to transform how we approach cancer treatment. Whether Ivermectin becomes a standard component of cancer care will depend on the willingness of the medical community to conduct the necessary research—and on patients and clinicians continuing to share their experiences through rigorous documentation.
What is clear from the available evidence is that Ivermectin’s anticancer mechanisms are biologically plausible, its safety profile is well-established, and preliminary clinical results are encouraging. These factors together make a compelling case for expanded investigation of ivermectin as an adjunctive cancer therapy, offering hope for more effective, accessible, and affordable cancer treatment options.
References
Arguello Cancer Clinic. (2015). *Atavistic chemotherapy: A study of anti-infective agents in the treatment of cancer* (Clinical trial registration No. NCT02366884). https://clinicaltrials.gov/study/NCT02366884
Crump, A., & Ōmura, S. (2011). Ivermectin, ‘wonder drug’ from Japan: The human use perspective. *Proceedings of the Japan Academy, Series B, Physical and Biological Sciences, 87*(2), 13-28. https://doi.org/10.2183/pjab.87.13
Dominguez-Gomez, G., Chavez-Blanco, A., Medina-Franco, J. L., Saldivar-Gonzalez, F., Flores-Torrontegui, Y., Juarez, M., Diaz-Chavez, J., Gonzalez-Fierro, A., & Dueñas-Gonzalez, A. (2018). Ivermectin as an inhibitor of cancer stem-like cells. *Molecular Medicine Reports, 17*(2), 3397-3403. https://doi.org/10.3892/mmr.2017.8231
Dou, Q., Chen, H. N., Wang, K., Yuan, K., Lei, Y., Li, K., Lan, J., Chen, Y., Huang, Z., Xie, N., Zhang, L., Xiang, R., Nice, E. C., Wei, Y., & Huang, C. (2016). Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. *Cancer Research, 76*(15), 4457-4469. https://doi.org/10.1158/0008-5472.CAN-15-2887
Guzzo, C. A., Furtek, C. I., Porras, A. G., Chen, C., Tipping, R., Clineschmidt, C. M., Sciberras, D. G., Hsieh, J. Y., & Lasseter, K. C. (2002). Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. *Journal of Clinical Pharmacology, 42*(10), 1122-1133. https://doi.org/10.1177/009127002401382731
Hashimoto, H., Messerli, S. M., Sudo, T., & Maruta, H. (2009). Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. *Drug Discoveries & Therapeutics, 3*(6), 243-246.
Hoffman, R. M., Han, Q., Murakami, T., Xu, M., Zhao, M., Bouvet, M., Yano, S., & Sugisawa, N. (2025). Ivermectin combined with recombinant methioninase (rMETase) synergistically eradicates MiaPaCa-2 pancreatic cancer cells. *Anticancer Research, 45*(1), 97-101. https://doi.org/10.21873/anticanres.16807
Jiang, L., Wang, P., Chen, L., Chen, H., Sun, Y. J., & Wu, Y. J. (2022). Ivermectin inhibits tumor metastasis by regulating the Wnt/β-catenin/integrin β1/FAK signaling pathway. *American Journal of Cancer Research, 12*(10), 4425-4442.
Juarez, M., Schcolnik-Cabrera, A., & Dueñas-Gonzalez, A. (2018). The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. *American Journal of Cancer Research, 8*(2), 317-331.
Kory, P. (2025). Case series of metastatic lung cancers treated with combination repurposed drug regimens. *Pierre Kory’s Medical Musings*. https://pierrekorymedicalmusings.com/p/case-series-of-metastatic-lung-cancers-eeb
Li, M. Y., Zhang, J., Lu, X., Zhou, D., Deng, X. F., Liu, Q. X., Dai, J. G., & Zheng, H. (2024). Ivermectin induces nonprotective autophagy by downregulating PAK1 and apoptosis in lung adenocarcinoma cells. *Cancer Chemotherapy and Pharmacology, 93*(1), 41-54. https://doi.org/10.1007/s00280-023-04589-6
Lu, Y., Li, C., Li, L., Wei, Q., Liu, Y., Zhou, P., Yang, X., Chen, L., Zhou, L., Liu, F., & Xiong, B. (2022). Ivermectin synergizes sorafenib in hepatocellular carcinoma via targeting multiple oncogenic pathways. *Pharmacology Research & Perspectives, 10*(3), e00954. https://doi.org/10.1002/prp2.954
Marik, P. E. (2024). *Cancer care: Repurposed drugs & metabolic interventions in treating cancer* (2nd ed.). Independent Medical Alliance. https://imahealth.org/research/cancer-care/
Melotti, A., Mas, C., Kuciak, M., Lorente-Trigos, A., Borges, I., & Ruiz i Altaba, A. (2014). The river blindness drug ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. *EMBO Molecular Medicine, 6*(10), 1263-1278. https://doi.org/10.15252/emmm.201404084
Napier, K. J., Scheerer, M., & Misra, S. (2020). Esophageal cancer: A review of epidemiology, pathogenesis, staging workup and treatment modalities. *World Journal of Gastrointestinal Oncology, 6*(5), 112-120. https://doi.org/10.4251/wjgo.v6.i5.112
Rujimongkon, K., Adchariyasakulchai, P., Meeprasertskul, P., & Ketchart, W. (2025). Ivermectin inhibits epithelial-to-mesenchymal transition via Wnt signaling in endocrine-resistant breast cancer cells. *PLOS ONE, 20*(6), e0326742. https://doi.org/10.1371/journal.pone.0326742
Tang, M., Hu, X., Wang, Y., Yao, X., Zhang, W., Yu, C., Cheng, F., Li, J., & Fang, Q. (2021). Ivermectin, a potential anticancer drug derived from an antiparasitic drug. *Pharmacological Research, 163*, 105207. https://doi.org/10.1016/j.phrs.2020.105207
Wang, K., Gao, W., Dou, Q., Chen, H., Li, Q., Nice, E. C., & Huang, C. (2016). Ivermectin induces PAK1-mediated cytostatic autophagy in breast cancer. *Autophagy, 12*(12), 2498-2499. https://doi.org/10.1080/15548627.2016.1231494
Yuan, J., Wang, L., & Chen, X. (2022). *Ivermectin and balstilimab or pembrolizumab in treating patients with metastatic triple-negative breast cancer* (Clinical trial registration No. NCT05318469). https://clinicaltrials.gov/study/NCT05318469
*Disclaimer: This article is for educational purposes only and should not be construed as medical advice. Cancer treatment decisions should be made in consultation with qualified oncology professionals. The case studies presented represent individual experiences and outcomes may vary. Patients should never discontinue or modify standard cancer treatments without consulting their healthcare team.*