From Chronic Fatigue To Cancer Risk: Understanding the IL-6 Connection in Post-Viral Syndromes – Part 1
Research in 2025-2026 has identified a critical inflammatory pathway shared across Long COVID, Post-Vaccine Syndrome, and even cancer progression: persistently elevated Interleukin-6 (IL-6). However, if you’ve been following us for a while, you know this research is finally catching up to what we have known from the beginning of the spike protein pandemic. If you read the research, you will hear how this discovery is transforming how conventional medicine understands and treats post-viral syndromes while revealing crucial connections to long-term health risks. At Leading Edge Clinic, where we’ve specialized in treating these conditions since 2022, we have been treating patients with the knowledge of IL-6’s role in post-viral syndromes all along. In this piece, we will talk about what new research gets right. Then, next week, we will cover what happens after elevated IL-6 is dealt with.
The IL-6 “Revolution”: A Paradigm Shift in Post-Viral Medicine
If you’re struggling with persistent fatigue, brain fog, or multi-system symptoms months or years after COVID-19 infection or vaccination, your body may be trapped in a state of chronic inflammation. The culprit? A single inflammatory molecule that’s emerging as the master orchestrator of post-viral syndromes: Interleukin-6.
Recent studies published in January 2026 have confirmed what clinicians treating Long COVID and Post-Vaccine Syndrome have long suspected, and even known: patients with persistent symptoms show sustained upregulation of inflammatory pathways, with IL-6 at the center of this dysfunction. This isn’t just academic knowledge. It is validation for patients, and hopefully something that will spur change in how more mainstream practitioners understand and treat these debilitating conditions.
But here’s what makes this research even more significant: the same IL-6 pathway implicated in post-viral syndromes is also a known driver of cancer progression. Understanding this connection isn’t just about treating today’s symptoms. It is about protecting your long-term health.
What Is IL-6 and Why Does It Matter?
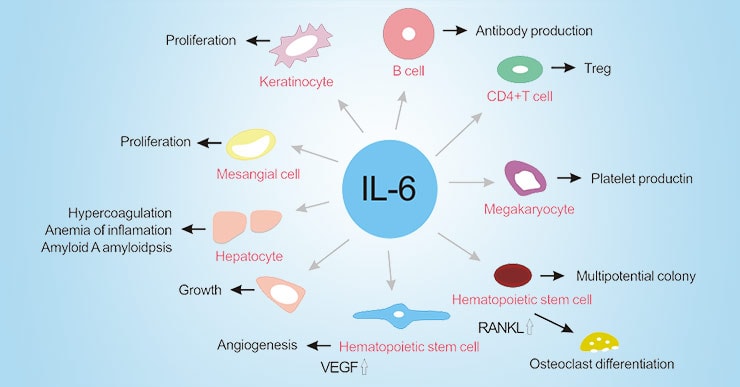
Interleukin-6 is a cytokine. Cytokines are signaling molecules your immune system uses to coordinate inflammatory responses. In healthy individuals, IL-6 spikes temporarily during infections or injury, helping your body fight off threats and heal damaged tissue. Once the threat is eliminated, IL-6 levels return to normal baseline.
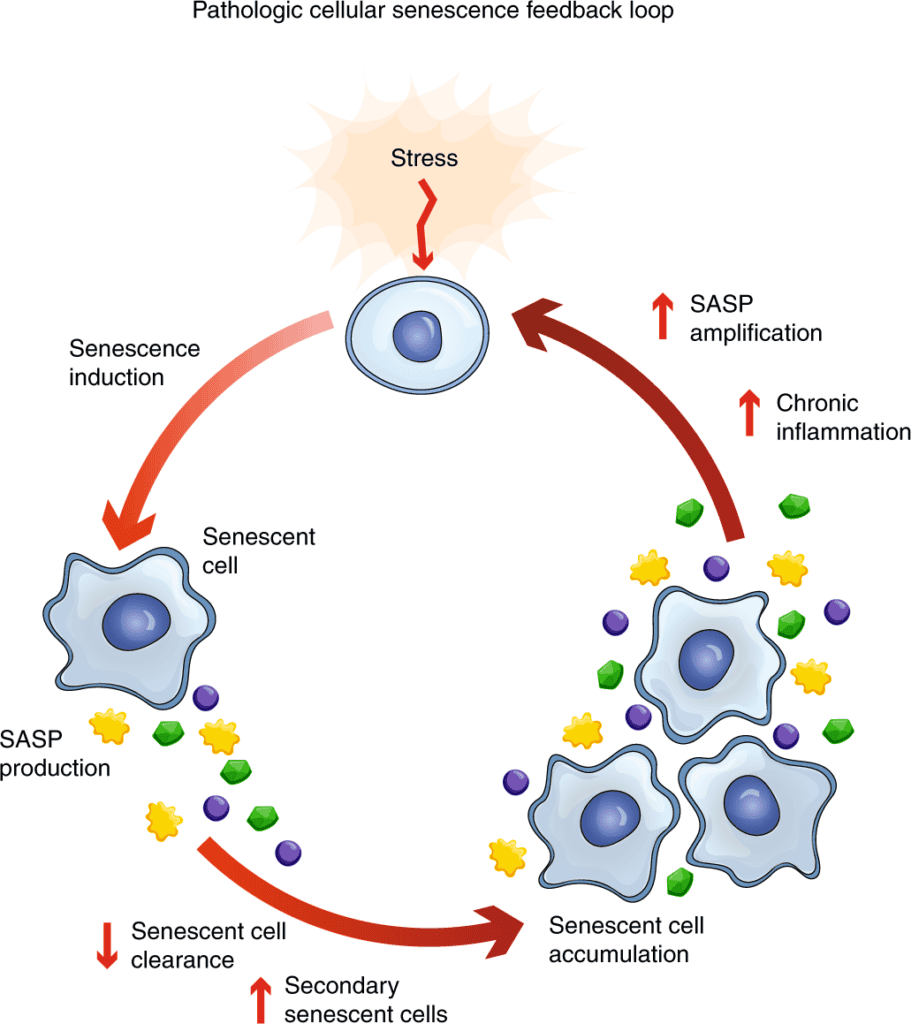
But in Long COVID, Post-Vaccine Syndrome, and certain chronic diseases, something goes wrong. Much of the current thinking is that IL-6 remains persistently elevated long after the initial trigger has resolved, creating a state of chronic, low-grade inflammation that damages tissues throughout your body. However, we believe it is more likely that the issue hasn’t fully resolved. This could be due to viral resevoirs, persistent spike production in the vaccine injured, immune dysregulation, etc…
What Elevated IL-6 Does to Your Body
Chronic IL-6 elevation isn’t benign. This persistent inflammatory signal creates a cascade of problems:
- Profound fatigue: IL-6 signals your brain to conserve energy, contributing to the overwhelming exhaustion characteristic of post-viral syndromes
- Cognitive dysfunction: Neuroinflammation driven by IL-6 contributes to brain fog, memory problems, and difficulty concentrating
- Muscle and joint pain: IL-6 promotes inflammatory pain pathways throughout your musculoskeletal system
- Immune dysregulation: Chronic IL-6 can exhaust certain immune cells while overactivating others, creating vulnerability to infections and autoimmunity
- Metabolic disruption: IL-6 interferes with insulin signaling and energy metabolism
- Cardiovascular stress: Promotes endothelial dysfunction and increases cardiovascular disease risk
- Cancer progression: Creates a pro-tumorigenic environment that can accelerate cancer development and growth
The Evidence: IL-6 in Long COVID and Post-Vaccine Syndrome
Long COVID: The IL-6 Signature
A January 2026 study published in Nature Immunology provided definitive evidence that Long COVID patients have sustained upregulation of chronic inflammatory pathways compared with people who fully recovered from SARS-CoV-2 infection. IL-6 emerged as one of the key differentiating markers.
Separate research from Cardiff Metropolitan University and Cwm Taf Morgannwg University Health Board identified elevated IL-6 levels in Long COVID patients compared to those who fully recovered. This finding has been replicated across multiple independent studies, establishing IL-6 elevation as one of the most consistent biomarkers in Long COVID.
Additional inflammatory markers frequently elevated alongside IL-6 in Long COVID patients include:
- IL-1β: Another pro-inflammatory cytokine that works synergistically with IL-6
- TNF-α: Tumor necrosis factor alpha, contributing to systemic inflammation
- IL-8: Involved in neutrophil recruitment and inflammation
These markers together paint a picture of a sustained inflammatory state that distinguishes Long COVID from normal post-infection recovery.
Post-Vaccine Syndrome: Parallel Inflammatory Patterns
Research on Post-Vaccine Syndrome (also called Post-Acute COVID-19 Vaccination Syndrome or PACVS) reveals similar inflammatory signatures. Studies analyzing blood markers in individuals with chronic symptoms following COVID-19 vaccination have consistently identified:
- Elevated IL-6 in over 80% of PACVS patients: A landmark German study found that more than 80% of individuals with Post-Vaccine Syndrome had increased IL-6 levels
- Elevated IL-8: Also present in over 80% of PACVS patients
- Altered receptor antibodies: Particularly changes in Angiotensin II type 1 receptor (AT1R) antibodies and alpha-2B adrenergic receptor antibodies
A February 2025 Yale University study on Post-Vaccination Syndrome identified similar immune dysregulation patterns, with researchers investigating IL-6 elevation alongside potential drivers including spike protein persistence, autoimmunity, tissue damage, and Epstein-Barr Virus (EBV) reactivation. Again, all things we have long known in our frontline experience treating the vaccine injured.
The Critical Finding: The combination of elevated IL-6, IL-8, and altered receptor antibodies can discriminate Post-Vaccine Syndrome from normal post-vaccination states with up to 90% accuracy, according to published research. This provides an objective diagnostic framework for a condition that has often been dismissed or misunderstood. Again, validation for patients who have been dismissed.
The Shared Pathophysiology: Why These Conditions Look So Similar
One of the most important clinical observations at Leading Edge Clinic has been the similarity between Long COVID and Post-Vaccine Syndrome presentations. We always had our hunches and detective skills telling us, but now we have research to back it: both conditions appear to involve persistent immune activation driven by similar inflammatory pathways, with IL-6 playing a central role. Vindication for us. But, more importantly, vindication for the spike portein injured.
The Spike Protein Connection
Emerging research suggests that in both conditions, the SARS-CoV-2 spike protein—whether from natural infection or vaccination—may persist longer than expected in some individuals. This persistence can trigger ongoing inflammatory responses. At the risk of sounding like a broken record… again, this is something we and many others could have definitively told anyone years ago. However, the research apparatus must confirm it via their gold standard methods!
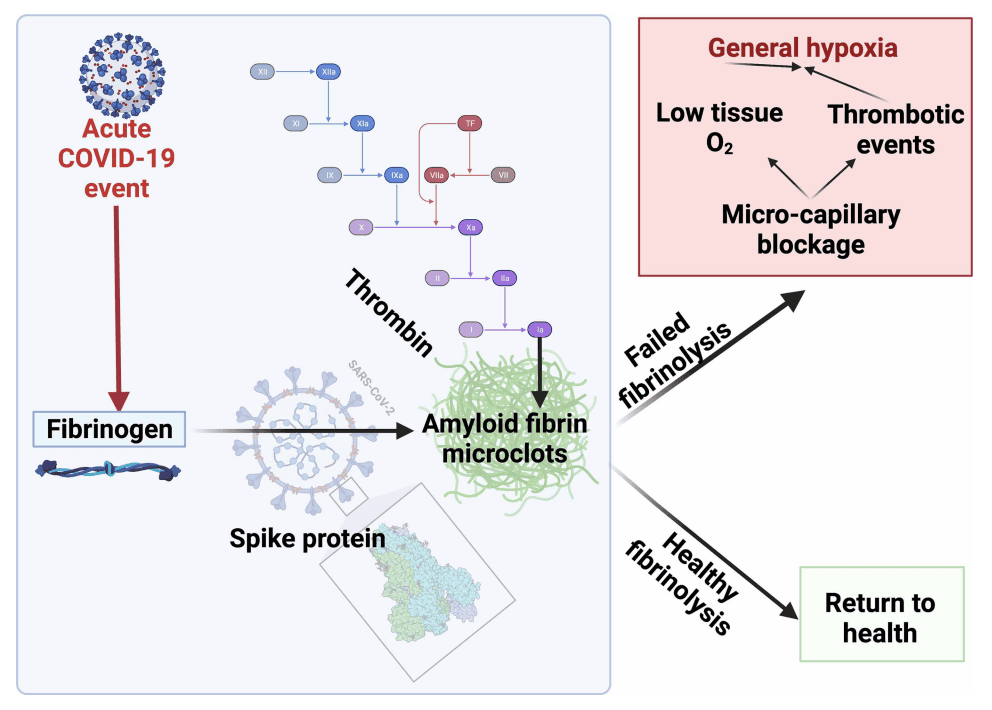
Research has found protein fragments from the COVID-19 virus hidden inside tiny cellular packages in the blood of Long COVID patients. The spike glycoprotein can:
- Induce endothelial inflammation and dysregulate coagulation pathways
- Alter mitochondrial function and increase reactive oxygen species
- Promote pro-inflammatory signaling in multiple organ systems
- Trigger sustained IL-6 production
This helps explain why both Long COVID and Post-Vaccine Syndrome share similar symptom profiles: fatigue, cognitive dysfunction, autonomic issues, and multi-system inflammation.
The Symptom Overlap
Both conditions frequently present with:
- Chronic fatigue and malaise: The most common symptom in both conditions
- Cognitive impairment: Brain fog, memory problems, difficulty concentrating
- Autonomic dysfunction: POTS symptoms, orthostatic intolerance, heart rate variability
- Peripheral neuropathy: Tingling, numbness, burning sensations; although we find this to be far more frequent in the vaccine injured population
- Sleep disorders: Despite exhaustion, restorative sleep remains elusive
- Gastrointestinal symptoms: Nausea, changes in bowel habits, abdominal discomfort
In fact, the majority of patients with Post-Vaccine Syndrome meet diagnostic criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), and many also fit criteria for POTS, fibromyalgia, and small fiber neuropathy—the same constellation seen in Long COVID.
Immune Exhaustion and Dysregulation
Beyond simple inflammation, both conditions show evidence of immune exhaustion—where certain immune cells become dysfunctional from chronic activation. This creates a paradox: patients are simultaneously hyperinflamed (elevated IL-6, IL-8) yet immunocompromised (exhausted T cells, poor response to new challenges).
The IL-6-Cancer Connection: Why This Matters for Long-Term Health
Here’s where the story becomes even more critical. The same IL-6 inflammatory pathway that’s chronically elevated in post-viral syndromes is also a well-established driver of cancer development and progression.
How IL-6 Promotes Cancer
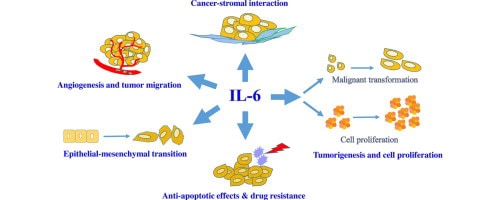
Oncology research has established that chronic inflammation, particularly IL-6-driven inflammation, creates a pro-tumorigenic environment through multiple mechanisms:
- Promoting cell proliferation: IL-6 activates signaling pathways (particularly STAT3) that encourage uncontrolled cell growth
- Inhibiting apoptosis: Cells that should die (including damaged or mutated cells) survive longer (more on this in part 2 of this series, which will be released next week)
- Promoting angiogenesis: New blood vessel formation that can feed growing tumors
- Facilitating metastasis: IL-6 helps cancer cells invade tissues and spread
- Suppressing anti-tumor immunity: Creates an immunosuppressive tumor microenvironment
- Inducing cachexia: The severe weight loss and muscle wasting seen in advanced cancer
High levels of IL-6 have been associated with poor prognosis in multiple cancer types, including breast, colorectal, lung, prostate, and ovarian cancers.
The Clinical Implications
What does this mean for people with Long COVID or Post-Vaccine Syndrome who have chronically elevated IL-6?
It means that addressing chronic inflammation isn’t just about feeling better today. It is also about protecting your long-term health.
While the mainstream research apparatus does not have long-term data on whether sustained IL-6 elevation from post-viral syndromes increases cancer risk specifically (this will require years of follow-up), we do know that:
- Chronic inflammation is an established cancer risk factor
- IL-6 specifically promotes cancer development and progression
- Reducing systemic inflammation reduces cancer risk in other contexts
- Patients with Long COVID or Post-Vaccine Syndrome deserve comprehensive care that addresses both current symptoms and future health risks
Testing for IL-6 and Inflammatory Markers
One of the most important developments in post-viral syndrome care is the ability to objectively measure inflammatory biomarkers. Not necessarily for treatment purposes, as we have been able to successfully treat regardless. But, more importantly, for the validation of patients ongoing suffering. This transforms these conditions from subjective, poorly understood syndromes into quantifiable medical conditions with measurable abnormalities. That makes it much more difficult for these patients to be ignored, dismissed, or told it is in their head.
Standard Blood Tests
Some tests that can identify these issues are:
- Serum IL-6 levels: Direct measurement of this key inflammatory cytokine
- High-sensitivity C-reactive protein (hs-CRP): A downstream marker of IL-6 activity and systemic inflammation
- IL-8 levels: Often elevated alongside IL-6 in post-viral syndromes
- Erythrocyte sedimentation rate (ESR): Another general inflammation marker
Note: at Leading Edge Clinic, we don’t need to order these tests in order to treat. Over and over again, our patients come in with hallmark symptoms of systemic inflammation. We find it financially draining, and energetically draining, to ask patients to drive to their local lab and have these tests done to provide us with an answer we already know. However, tracking can be useful to understand progress.
Advanced Biomarker Panels
Some more advanced testing may include:
- Receptor autoantibodies: Including AT1R and adrenergic receptor antibodies shown to distinguish Post-Vaccine Syndrome
- Cytokine panels: Measuring multiple inflammatory markers including TNF-α, IL-1β, and others
- Markers of immune exhaustion: To understand the full immune dysfunction picture
- Free T3 thyroid hormone: Low free T3 found in over 80% of Post-Vaccine Syndrome patients
- IgG subclass analysis: Imbalances present in over 50% of patients
- Soluble neurofilament light chains: Marker of neurological damage, elevated in about 30% of patients
These objective markers provide validation for patients whose symptoms have been dismissed and guide targeted treatment strategies. We are more likely to order these, as they can actually guide treatment.
Targeting IL-6: Evidence-Based Treatment Strategies
Understanding that IL-6 and chronic inflammation are central to these conditions opens new therapeutic possibilities. The goal is to normalize inflammatory pathways while supporting your body’s natural healing mechanisms.
Pharmaceutical Approaches
Direct IL-6 Inhibitors:
In conventional medicine, medications like tocilizumab directly block IL-6 signaling and are FDA-approved for conditions like rheumatoid arthritis. While not yet standard of care for post-viral syndromes, research is evaluating whether these agents could benefit severe cases with markedly elevated IL-6. However, we believe there are much safer, low-cost, and more effective therapies, such as the one listed next.
Low-Dose Naltrexone (LDN):
LDN has long been a mainstay of treatment, due to its ability to modulate inflammatory pathways and reduce IL-6 production. Many patients with post-viral syndromes report symptom improvements with LDN, and it has an excellent safety profile. It acts directly on production of IL-6 in the liver.
Natural Anti-Inflammatory Interventions
Several natural compounds have demonstrated ability to reduce IL-6 production and signaling:
- Omega-3 fatty acids (EPA/DHA): High-dose fish oil (2-4 grams daily) has been shown to reduce IL-6 and other inflammatory markers. Choose pharmaceutical-grade products to avoid contaminants.
- Curcumin: The active compound in turmeric potently inhibits IL-6 production. Use enhanced bioavailability formulations (with piperine or liposomal delivery) at doses of 500-2000mg daily.
- Resveratrol: Found in grape skins and Japanese knotweed, resveratrol suppresses IL-6 signaling pathways. Typical doses: 200-500mg daily.
- Quercetin: A flavonoid with anti-inflammatory and antiviral properties that can reduce IL-6. Dose: 500-1000mg daily.
- Green tea extract (EGCG): Epigallocatechin gallate modulates inflammatory pathways including IL-6. Dose: 400-800mg daily.
- Specialized pro-resolving mediators (SPMs): These omega-3 derivatives actively resolve inflammation rather than just suppressing it.
Note: a lof of these therapies have blood-thinning properties. Additionally, depending on how you are presenting as a Long Covid or Post-Vaccine Syndrome patient, some of these therapies may be innapropriate (ie: if Mast Cell Activation Syndrome is indicated). We recommend working with a clinician knowledgable in spike protein conditions.
Lifestyle Interventions
- Anti-inflammatory diet: Mediterranean-style eating rich in vegetables, fruits, olive oil, fish, nuts, and whole grains while minimizing processed foods, sugar, and inflammatory oils
- Intermittent fasting: Time-restricted eating and intermittent fasting can promote autophagy and reduce inflammatory markers
- Sleep optimization: Poor sleep drives IL-6 production; prioritizing restorative sleep is crucial
- Stress management: Chronic psychological stress elevates IL-6; mind-body practices like meditation can help
- Appropriate exercise: While overexertion worsens symptoms, appropriate gentle movement within energy limits can help regulate inflammation
Addressing Root Causes
Beyond symptomatic IL-6 reduction, we must address what’s driving the chronic inflammation:
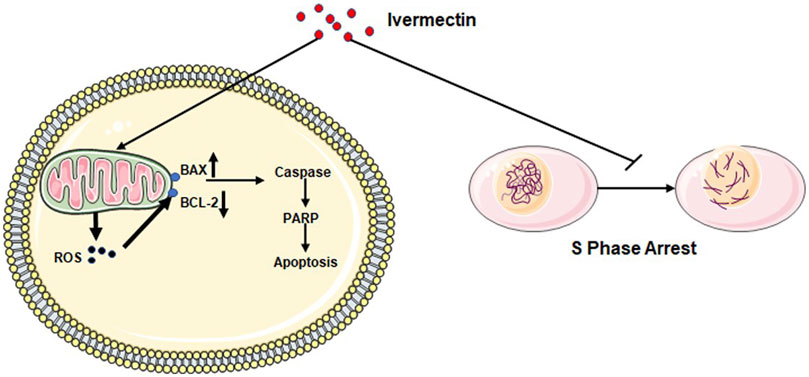
- Spike protein clearance: There are a number of ways to potentially clear spike protein. This is tricky because of its immune evasion. It also uses commensal gut bacteria as bacteriophages, making it even more difficult to get rid of. Some ways are: supporting autophagy through intermittent fasting, certain supplements, and potentially medications like ivermectin may help clear persistent viral proteins
- Microclot dissolution: For patients with evidence of microclotting, carefully monitored anticoagulation and/or antiplatelet strategies
- Immune rebalancing: Using immune-modulating agents to restore normal immune function rather than simply suppressing inflammation
- Gut microbiome restoration: The gut-immune axis plays a crucial role in systemic inflammation
- Mitochondrial support: Addressing energy metabolism dysfunction
Cancer Prevention Considerations
Given the IL-6-cancer connection, patients with chronic post-viral syndromes should be particularly attentive to cancer prevention strategies:
Anti-Cancer Lifestyle
- Maintain healthy body weight: Excess adipose tissue produces IL-6 and other inflammatory cytokines
- Minimize alcohol: Alcohol increases inflammation and cancer risk
- Minimize processed foods: Highly processed foods contribute to inflammation and cancer risk
- Don’t smoke: Smoking dramatically increases both inflammation and cancer risk
- Eat cruciferous vegetables: Broccoli, cauliflower, and Brussels sprouts contain compounds that support detoxification
- Optimize vitamin D: Maintain levels between 80 ng/mL+ for immune and anti-cancer benefits
Repurposed Medications with Anti-Cancer Properties
Some medications used in post-viral syndrome treatment also have documented anti-cancer effects:
- Metformin: Being studied for Long COVID prevention and has established anti-cancer properties
- Ivermectin: Aside from its interference with various cell signaling pathways, its anti-inflammatory effects may provide some cancer protection
- Low-dose aspirin: Reduces colorectal cancer risk (discuss with your physician)
Note: There are many other evidence-based repurposed drug therapies we utilize in our adjunctive cancer care practice.
Leading Edge Clinic’s Comprehensive Approach
At Leading Edge Clinic, we’ve been at the forefront of treating Long COVID and Post-Vaccine Syndrome since 2022. Our approach is built on not only scientific understanding of IL-6 and inflammatory pathways, but years of clinical experience at the frontlines treating these novel conditions
Step 1: Comprehensive Assessment
- Detailed symptom history and timeline
- Review of spikopathy and correlation to symptom onset and overall condition
- If there is an appetite for testing: Comprehensive inflammatory marker testing including IL-6, IL-8, hs-CRP; receptor autoantibody panels when indicated; Thyroid function, IgG subclasses, and other relevant functional markers
- Discussion on evidence-based treatments available
Step 2: Personalized Treatment Protocol
Based on your specific inflammatory profile and symptom presentation, we develop a targeted protocol that may include:
- Anti-inflammatory pharmaceutical agents when appropriate
- Evidence-based nutraceutical protocols targeting IL-6 and chronic inflammation
- Interventions to address spike protein persistence and microclotting
- Immune rebalancing strategies
- Mitochondrial and metabolic support
- Personalized lifestyle and dietary recommendations
Step 3: Ongoing Monitoring and Adjustment
- Proactive medical staff follow-up to track symptom improvement
- Protocol adjustments based on response
- Long-term health optimization
Our goal isn’t just symptom management—it’s helping your body restore normal inflammatory balance and protecting your long-term health.
Why This Knowledge Matters: A Patient’s Perspective
If you’re living with Long COVID or Post-Vaccine Syndrome, understanding the IL-6 connection provides several crucial benefits:
- Validation: Your symptoms have objective, measurable biological underpinnings. This isn’t in your head.
- Targeted treatment: Knowing the mechanism allows for specific interventions
- Monitoring progress: IL-6 levels can be tracked over time, if there is patient appetite for ongoing testing
- Long-term health protection: Understanding the cancer connection motivates comprehensive anti-inflammatory strategies
Conclusion: Knowledge Is Validation
The discovery that IL-6 elevation is a shared feature of Long COVID, Post-Vaccine Syndrome, and cancer progression represents objective and measurable biological changes that patients can point to. While we have already been using this as a roadmap for effective intervention since 2022, academics now catching up provides validation for patients’ suffering.
The connection between chronic inflammation and cancer risk underscores why addressing post-viral syndromes isn’t just about quality of life today—it’s about protecting your health for years to come.
At Leading Edge Clinic, we’re committed to translating clinical observations into practical treatment protocols that make a real difference in patients’ lives, even if the research is years behind. We don’t just treat symptoms—we address the underlying inflammatory pathways driving your condition.
Take Control of Your Inflammation
If you’re experiencing persistent symptoms after COVID-19 infection or vaccination, don’t wait. Early intervention to address chronic inflammation can prevent long-term complications and improve your quality of life.
Contact Leading Edge Clinic today to schedule a comprehensive assessment and develop your personalized treatment protocol.
Our team specializes in Long COVID, Post-Vaccine Syndrome, and the inflammatory pathways that connect them. We offer telehealth consultations, making expert care accessible from anywhere.
It Is About More Than Just Inflammation
In part two of this series, we will draw the connection between IL-6 and heightened inflammatory states, to chronic Cell Danger Response. Addressing systemic inflammation is critical, but it isn’t the only piece of the puzzle. Chronic Cell Danger Response is a key factor in ongoing illness, and takes time and patience to address. Be on the lookout for this blog post on Tuesday of next week.
Key Takeaways
- IL-6 elevation is a consistent finding in both Long COVID and Post-Vaccine Syndrome, providing objective biomarker evidence for these conditions
- Chronic IL-6 elevation drives the fatigue, cognitive dysfunction, and multi-system symptoms characteristic of post-viral syndromes
- The same IL-6 pathway promotes cancer development and progression, making inflammation control crucial for long-term health
- Testing for IL-6, IL-8, and related markers provides objective diagnosis and treatment monitoring
- Multiple evidence-based strategies can reduce IL-6 levels, from pharmaceutical interventions to natural anti-inflammatory compounds
- Specialized care addressing inflammatory pathways offers better outcomes than generic approaches
References and Further Reading
Key Research Sources:
- Nature Immunology (January 2026) – Chronic inflammatory pathways in Long COVID
- Cardiff Metropolitan University & Cwm Taf Morgannwg University Health Board – IL-6 elevation in Long COVID
- Yale University LISTEN Study (February 2025) – Post-Vaccination Syndrome biomarkers
- Vaccines Journal (July 2024) – Clinical and diagnostic features of PACVS including IL-6/IL-8 findings
- PMC Studies on receptor antibodies and inflammation in Post-Vaccine Syndrome
- Cancer research literature on IL-6’s role in tumorigenesis and progression
Disclaimer:
This article is for educational and informational purposes only and is not intended as medical advice, diagnosis, or treatment. Always consult qualified healthcare providers for diagnosis and treatment of medical conditions. Individual responses to treatment vary, and what works for one person may not work for another. Treatment decisions should be made in consultation with healthcare providers familiar with your complete medical history. Leading Edge Clinic provides this information to empower patients with knowledge while emphasizing the importance of professional medical guidance.